Technetium immobilization by chloride green rust
Mayordomo, N1, Rodríguez, D. M.1, Schild, D.2, Rossberg, A.1,3, Scheinost, A. C.1,3, Brendler, V.1, Müller, K.1
1 Institute of Resource Ecology, Helmholtz-Zentrum Dresden – Rossendorf (HZDR), 01328 Dresden (Germany). 2 Institute for Nuclear Waste Disposal, Karlsruhe Institute of Technology (KIT), 76344, Eggenstein-Leopoldshafen (Germany). 3 The Rossendorf Beamline at ESRF (ROBL), 38043, Grenoble (France).
INTRODUCTION
Technetium-99 (99Tc) is one of the most concerning fission products due to its long half-life (2.14∙105 years) and the high mobility of the anion pertechnetate (TcO4-). [1]
Tc migration decreases when Tc(VII) is reduced to Tc(IV). This scavenging step is carried out by Fe(II) minerals, which have been widely studied due to their versatility, low cost and ubiquity. [2] Green rust is a Fe(II)-Fe(III) hydroxide that possesses adsorption, anion exchange and reduction capabilities. Its presence is expected in the near- and far-field of a nuclear waste repository because it is an iron corrosion product, and it is also formed in the environment when Fe2+ interacts with Fe(III) minerals. [3] Thus, further studies are needed to both identify the optimal Tc scavenging conditions by green rust and the mechanism responsible of Tc retention.
DESCRIPTION OF THE WORK
Batch contact studies have been performed under a wide range of conditions, i.e. pH (3-11), Tc concentration (nM-mM), and ionic strength (0-0.1 M). X-ray powder diffraction, Raman microscopy, X-ray photoelectron spectroscopy (XPS), and X-ray absorption spectroscopy (XAS) provided information on Tc oxidation state and speciation as well as on secondary redox products related to the Tc interaction with chloride green rust (GR(Cl)). In addition, re-oxidation experiments have been performed for one year to analyze the Tc retention reversibility.
RESULTS AND DISCUSSION
The results show that GR(Cl) removes Tc from solution with efficiencies between 80% (Kd = 8.0∙103 mL/g) and ≈100% (Kd = 9.9∙105 mL/g) for pH > 6.0.
In contrast, Tc removal for pH < 6.0 drops with decreasing pH, and ranges from 80% to 50% (Kd = 2.0∙103 mL/g), reaching a minimum at pH 3.5. XPS analysis reveals the predominance of Tc(IV) at all evaluated pH values (3.5 to 11.5), supporting that Tc reductive immobilization is the main retention mechanism. Re-oxidation experiments show that Tc is slowly solubilized when time increases.
The analysis of the extended X-ray absorption fine structure (EXAFS) reveals a change on Tc(IV) environment depending on pH and Tc loading. The most probable structural rearrangements are represented by Tc(IV) sorption on Fe(III) minerals formed as secondary phases with Tc polynuclear species contribution.
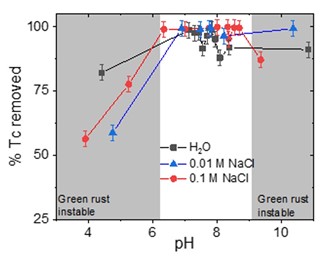
Fig. 1: Tc removal (%) by GR(Cl) as a function of pH for different ionic strengths (square) H2O, (triangle) 0.01 M NaCl and (circle) 0.1 M NaCl.
We thank the German Federal Ministry of Economic Affairs and Energy (BMWi) for funding the VESPA II project (02E11607B).
REFERENCES
1. MEENA, A.H.; ARAI, Y. "Environmental geochemistry of technetium" Env. Chem Lett, 15, 241–263 (2017).
2. PEARCE, C.I. et al., "Technetium immobilization by materials through sorption and redox-driven processes: A literature review" Sci. Total Environ,. 716, 132849 (2020).
3. USMAN, M.; et al., "Magnetite and Green Rust: Synthesis, Properties, and Environmental Applications of Mixed-Valent Iron Minerals" Chem. Rev. 118, 3251–3304, (2018).



